Breakthrough Cases and Boosters: Vaccine Strategies
Disclaimer: This resource addresses vaccination policies and federal law. Additionally state legislatures and state regulatory bodies may issue or enact laws, rules, directives, etc. that limit what your organization can do. It is imperative you have written policies reviewed and approved by legal counsel prior to implementation. Do not use this article for your regulatory compliance or healthcare guidance without consulting your attorney. None of the information we provide is intended as legal or medical advice.
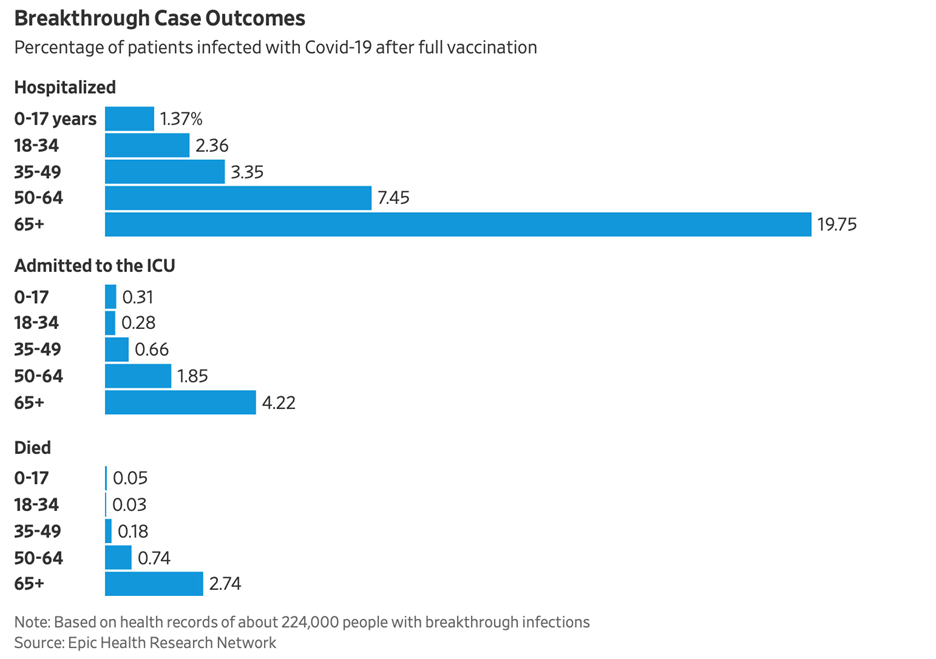
Article Quick Summary: Vaccinations for senior citizens over the age of 80 are losing their effectiveness, due to the Delta variant and waning efficacy due to the passage of time. The result is increasing breakthrough cases for vaccinated senior citizens, leading to disproportionate hospitalizations and deaths. Senior citizens should strongly consider using the booster, on advice of their physicians, to combat COVID resurgence.
From a November, 21, 2021 article in the Wall Street Journal (WSJ):
“Breakthrough deaths are hitting older people the hardest, amplifying a well-worn pandemic pattern. Exclusive data the Journal reviewed from the Epic Health Research Network, which analyzes data from the medical-record software company Epic Systems Corp., shows about 80% of breakthrough deaths (emphasis added) among the vaccinated are in people ages 65 and older.”1
As was the case before the vaccinations rolled out, breakthrough cases of COVID are most deadly to our senior residents and friends.1
A breakthrough case is when someone contracts COVID, but they have been given both vaccinations and at least 14 days have passed since they received the last vaccination shot.
As the chart shows above, roughly 34% of hospitalizations are now breakthrough cases. Delta and the passage of time has made the vaccinations weaker in preventing people from contracting the disease. However, vaccinated individuals are still much better protected from death, should they contract the virus, according to the CDC.2
The timing tipping point, when immunity seems to plunge after the vaccines, is 20-22 weeks. Given that most seniors in our communities completed their vaccine regimens by the end of April 2021, their immunity is now in the plunging category post-September 2021. Any remaining holdouts on the booster should strongly be encouraged to have the booster shot.3
The New York Times, quoting an Israeli study, notes that, “At least 12 days after the booster, rates of infection were eleven fold lower and of severe disease nearly twenty fold lower in those who received a booster compared with those who had received only two doses, the researchers found.”4
However, most scientists agree that this increased efficacy, like the original vaccine dosages, will wane. The timing of this drop in efficacy is probably 3-4 months after receiving the booster. 4
Most physicians believe there is limited to zero value to receive boosters for individuals under the age of 65 with no medical complications.4
For most people, their immune response to the vaccinations is adequate to protect against hospitalization, at least for the short-term future.
So, what to do for seniors?
Based on current evident, the safest track is to:
- Strongly encourage booster vaccines for your residents
- Make boosters easy (host a “booster day” for residents with a local clinic)
- Plan a cycle of boosters every 4-5 months, to provide protection from Delta and other variants of COVID
- Stay tuned into additional regulations and guidance by the government and local authorities
Disclaimer: This resource addresses vaccination policies and federal law. Additionally state legislatures and state regulatory bodies may issue or enact laws, rules, directives, etc. that limit what your organization can do. It is imperative you have written policies reviewed and approved by legal counsel prior to implementation. Do not use this article for your regulatory compliance or healthcare guidance without consulting your attorney. None of the information we provide is intended as legal or medical advice.
Ready to Talk?
- https://www.wsj.com/articles/covid-19-breakthrough-hospitalizations-concentrated-among-most-vulnerable-11637499602?mod=hp_lead_pos5
- https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/why-measure-effectiveness/breakthrough-cases.html
- https://www.nature.com/articles/d41586-021-02532-4
- https://www.nytimes.com/2021/09/15/health/covid-booster-shot-data.html